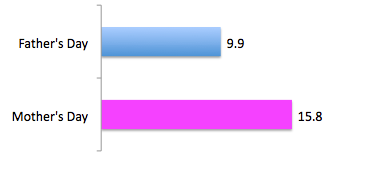
An increase in MMP3 was found in IPF lung tissue. Mmmp3–/– mice were protected from bleomycin induced lung fibrosis, through beta-catenin mediated EMT.
References:
Am J Pathol. 2011 Oct;179(4):1733-45. Epub 2011 Aug 24.
Matrix metalloproteinase 3 is a mediator of pulmonary fibrosis.
Yamashita CM, Dolgonos L, Zemans RL, Young SK, Robertson J, Briones N, Suzuki T, Campbell MN, Gauldie J, Radisky DC, Riches DW, Yu G, Kaminski N, McCulloch CA, Downey GP.
Source
Department of Medicine, University of Western Ontario, London, Ontario, Canada; Division of Pulmonary and Critical Care Medicine, Departments of Medicine and Pediatrics, National Jewish Health, Denver, Colorado.
Abstract
Idiopathic pulmonary fibrosis (IPF) may be triggered by epithelial injury that results in aberrant production of growth factors, cytokines, and proteinases, leading to proliferation of myofibroblasts, excess deposition of collagen, and destruction of the lung architecture. The precise mechanisms and key signaling mediators responsible for this aberrant repair process remain unclear. We assessed the importance of matrix metalloproteinase-3 (MMP-3) in the pathogenesis of IPF through i) determination of MMP-3 expression in patients with IPF, ii) in vivo experiments examining the relevance of MMP-3 in experimental models of fibrosis, and iii) in vitro experiments to elucidate possible mechanisms of action. Gene expression analysis, quantitative RT-PCR, and Western blot analysis of explanted human lungs revealed enhanced expression of MMP-3 in IPF, compared with control. Transient adenoviral vector-mediated expression of recombinant MMP-3 in rat lung resulted in accumulation of myofibroblasts and pulmonary fibrosis. Conversely, MMP-3-null mice were protected against bleomycin-induced pulmonary fibrosis. In vitro treatment of cultured lung epithelial cells with purified MMP-3 resulted in activation of the β-catenin signaling pathway, via cleavage of E-cadherin, and induction of epithelial-mesenchymal transition. These processes were inhibited in bleomycin-treated MMP-3-null mice, as assessed by cytosolic translocation of β-catenin and cyclin D1 expression. These observations support a novel role for MMP-3 in the pathogenesis of IPF, through activation of β-catenin signaling and induction of epithelial-mesenchymal transition.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
PMID: 21871427 [PubMed - in process] PMCID: PMC3181358 [Available on 2012/10/1]
References:
Am J Pathol. 2011 Oct;179(4):1733-45. Epub 2011 Aug 24.
Matrix metalloproteinase 3 is a mediator of pulmonary fibrosis.
Yamashita CM, Dolgonos L, Zemans RL, Young SK, Robertson J, Briones N, Suzuki T, Campbell MN, Gauldie J, Radisky DC, Riches DW, Yu G, Kaminski N, McCulloch CA, Downey GP.
Source
Department of Medicine, University of Western Ontario, London, Ontario, Canada; Division of Pulmonary and Critical Care Medicine, Departments of Medicine and Pediatrics, National Jewish Health, Denver, Colorado.
Abstract
Idiopathic pulmonary fibrosis (IPF) may be triggered by epithelial injury that results in aberrant production of growth factors, cytokines, and proteinases, leading to proliferation of myofibroblasts, excess deposition of collagen, and destruction of the lung architecture. The precise mechanisms and key signaling mediators responsible for this aberrant repair process remain unclear. We assessed the importance of matrix metalloproteinase-3 (MMP-3) in the pathogenesis of IPF through i) determination of MMP-3 expression in patients with IPF, ii) in vivo experiments examining the relevance of MMP-3 in experimental models of fibrosis, and iii) in vitro experiments to elucidate possible mechanisms of action. Gene expression analysis, quantitative RT-PCR, and Western blot analysis of explanted human lungs revealed enhanced expression of MMP-3 in IPF, compared with control. Transient adenoviral vector-mediated expression of recombinant MMP-3 in rat lung resulted in accumulation of myofibroblasts and pulmonary fibrosis. Conversely, MMP-3-null mice were protected against bleomycin-induced pulmonary fibrosis. In vitro treatment of cultured lung epithelial cells with purified MMP-3 resulted in activation of the β-catenin signaling pathway, via cleavage of E-cadherin, and induction of epithelial-mesenchymal transition. These processes were inhibited in bleomycin-treated MMP-3-null mice, as assessed by cytosolic translocation of β-catenin and cyclin D1 expression. These observations support a novel role for MMP-3 in the pathogenesis of IPF, through activation of β-catenin signaling and induction of epithelial-mesenchymal transition.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
PMID: 21871427 [PubMed - in process] PMCID: PMC3181358 [Available on 2012/10/1]